Startup Jedi
We talk to startups and investors, you get the value.

Startup Jedi
We talk to startups and investors, you get the value.
AI greatly influences medicine and health care delivery. Despite the fact that there is still lack of evidence for the use of AI in medicine, there are numerous practical examples of implementing it. AI technologies demonstrate the potential for diagnosing, managing and treating a wide range of diseases. Yet, there are many obstacles to introducing AI startups into everyday clinical practice, especially with regard to regulating them.
We talked about software as a medical device (SaMD) and its significance in the MedTech field, as well as how the Pre-Cert program allows you to quickly bring a SaMD product to market in the 1st part of the series. The 2nd part covered the advantages of using SaMD in medicine, practical examples, clinical use cases, and further prospects for its development. The third part of SaMD articles focuses on AI-based startups that are certified and approved by the US Food and Drug Administration (FDA).

In 2020 Nature's npj Digital Medicine described 64 FDA-approved medical devices and algorithms using AI, and in 2021 The Lancet Digital Health described 222 medical AI-based devices approved in the US and Europe.
AI tech has clinical value for radiology, oncology, ophthalmology and general medical decision making. It has been proven that AI-based solution models reduce waiting times, improve adherence to medication, allow for proper insulin dosage, and aid in the interpretation of magnetic resonance imaging.
The two main specialties with AI-driven medical innovations are radiology and cardiology, with twenty-one (72.4%) and four (13.8%) FDA-approved medical devices and algorithms, respectively. The rest are grouped as focused on therapy, endocrinology, neurology, ophthalmology, emergency medicine and oncology.
The medical field of radiology is a trendsetter in FDA-approved medical devices and algorithms, with the implementation of AI-based solutions for image-reading application software. Examples include the three algorithms or Arterys Inc., Arterys Cardio DL, Arterys Oncology DL, and Arterys MICA, which are connected to image archiving and workflow communication systems from major vendors such as Siemens Healthineers AG (Germany) and GE Healthcare (USA).
Six of these 21 algorithms can be applied in oncology, followed by two algorithms focused on brain imaging analysis, with innovations for the detection of stroke and hemorrhage in neurology. Four more algorithms focused on emergency care, with two algorithms for assessing pneumothorax, one focused on diagnosing wrist fractures and the Aidoc Medical BriefCase system for grading head, spine, and chest injuries.
Since diabetes has affected a large segment of society, innovations in blood glucose management are highly justified as well. The first steps were taken with the introduction of Guardian Connect by Medtronic and DreaMed Diabetes. AI-based interpretation of laboratory results has also been introduced for therapy with the FerriSmart assay system to assess liver iron concentration.

Despite numerous advantages, there are a number of barriers to implementing AI solutions in everyday clinical practice.
Due to the high risk and unknown implications of AI for medical decision making and data analysis, the FDA has regulatory requirements for the licensing of medical devices. Developers of medical devices and AI-based algorithms must go through certification stages. This can be considered a key barrier to the introduction of AI into medicine.
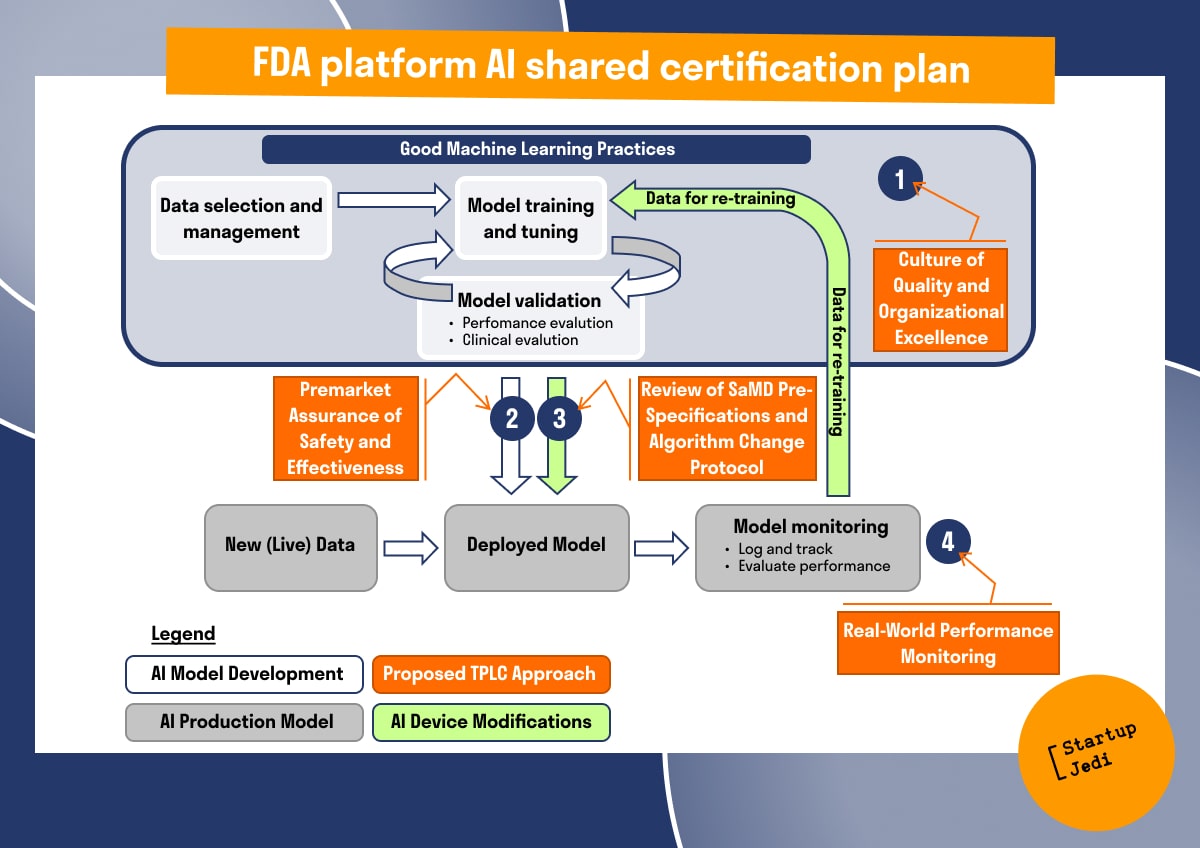
FDA Platform AI Shared Certification Plan
The FDA approval certification is a set of specific tests covering various parameters to be assessed. These include 510 (k) pre-sale notice, PMA pre-sale approval and de novo. Each of these types of tests allows the FDA to properly assess product compliance and provide marketing rights to the applicant.
Pre-Sale Notice 510 (k) is a preliminary filing with the FDA to certify that a device to be launched on the market is at least as safe and effective (nearly equivalent) to the devices already marketed and is not subject to PMA. In fact, it is the FDA's process for evaluating the safety and efficacy of medical devices.
Pre-Sale Approval (PMA) is the FDA's scientific and regulatory review process to assess the safety and efficacy of Class III medical devices (life-supporting devices that are essential to prevent human health deterioration or present a potential undue risk of illness or injury) and the most rigorous of device marketing applications. PMA approval usually requires inspecting the facility.
De novo path to gain device marketing rights has been added for low to medium risk products. After successful review of the de novo application, the FDA will create a classification for the device, if necessary, rules for its operation, and determine any special measures necessary for future pre-marketing activities with equivalent devices. Devices that are classified through a de novo process can be marketed and used as predicates to obtain 510 (k).
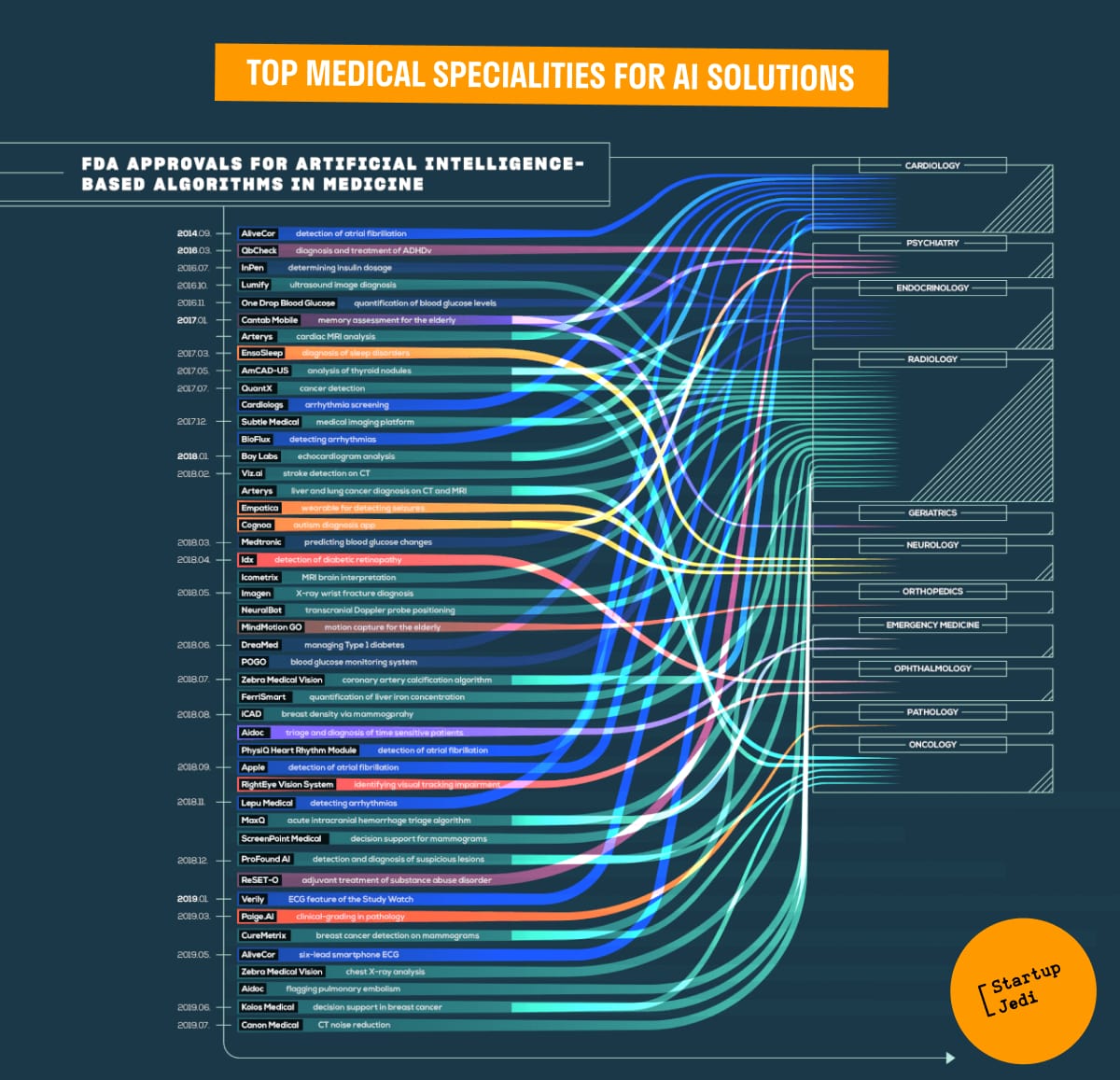
Source:The-Medical-Futurist-FDA-approved-AI-algorithms-in-medicine-2019 (medicalfuturist.com)
The infographic by FDA clearly shows the distribution of AI-based products in various medical specialties. Radiology and cardiology are the leading ones with 7 approved algorithms in cardiology and 16 in radiology.
These two medical specialties represent peak areas of AI research for several reasons. First of all, computer vision is one of the fastest growing areas in AI development, and medical imaging has both the data and the visibility that algorithms need to develop. Software to automatically screen for breast density and thus detect breast cancer could work on par with radiologists, the study said.
What's more, in April 2018, the FDA approved the first AI system that can be used for medical diagnostics without a doctor.

AliveCor has developed an ECG analytic platform providing early detection of atrial fibrillation;
QbCheck helps with the diagnosis and treatment of attention deficit hyperactivity disorder;
InPen monitors insulin dosage;
One Drop Blood Glucose quantifies your blood glucose and automatically sends the data to the paired app;
Lumify offers ultrasound imaging diagnostics;
Cantab Mobile acts as a tool for assessing memory problems in the elderly;
EnsoSleep provides automated assessment and analysis of sleep disorders;
AmCAD-US evaluates thyroid nodules and classifies nodule characteristics;
Lepu Medical and Bioflux detect arrhythmias;
Subtle Medical offers a medical imaging platform;
Bay Labs offers echocardiogram analysis;
Viz.AI detects stroke on CT;
Arterus is able to detect cancers in the liver and lungs on CT and MRI scans;
Empatica analyzes epileptic seizures;
The Cognoa algorithm built into the app helps diagnose autism in children;
Medtronic and POGO monitor and predict changes in blood glucose levels;
Icometrix helps neurologists interpret MR images of the brain;
Imagen is involved in the detection of wrist fractures;
DreaMed Diabetics helps healthcare providers treat type 1 diabetes;
Zebra Medical Vision quantifies coronary artery calcification and analyzes chest X-rays;
Aidoc is able to analyze brain hemorrhages on CT scans of the head and pulmonary embolism;
iCAD detects breast cancer;
ScreenPoint Medical assists radiologists with mammogram analysis;
RightEye Vision tracks eye movements to detect optic nerve disorders;
MaxQ develops an algorithm for the classification of acute intracranial hemorrhages;
ProFound AI detects and diagnoses breast lesions;
ReSET/ReSET-O is used in the treatment of substance and opioid use disorders;
Verily has developed an algorithm for continuous ECG monitoring;
Paige.AI — algorithm for the analysis of clinical images in pathologonatomy;
FerriSmart is a solution for quantifying liver iron concentration.

Every AI technology for healthcare needs to be regulated, effective and evidence-based. The US Food and Drug Administration (FDA) provides an example of creating a regulatory environment that not only welcomes such innovations, but can also make them safe for the public.
This could potentially lead to rules that allow regulators to rate companies, while companies can deploy algorithms and updates without having to test all of them. This viable method will allow AI-based technologies to spread, while ensuring their safety.
It is especially worth noting that AI-based technologies for healthcare can only be effective if they are successfully introduced into medical practice. The American Medical Association (AMA) has advocated the use of AI technology in medicine and has become a thought leader, including reporting and guidelines for physicians.
Research on the potential and problems of AI technologies, the creation of algorithms and their certification are the basis for improving medical care quality.
Sources: World Health Organization. Big data and artificial intelligence, pubmed, globenewswire.com, orthogonal.io, The Medical Futurist, The Lancet Digital Health, Software as a Medical Device Market (theinsightpartners.com), The state of artificial intelligence-based FDA-approved medical devices and algorithms: an online database | npj Digital Medicine (nature.com), FDA. Proposed regulatory framework for modifications to artificial intelligence/machine learning (AI/ML)-based software as a medical device (SaMD) — Discussion Paper and Request for Feedback.
Facebook: facebook.com/StartupJedi/
Telegram: t.me/Startup_Jedi
Twitter: twitter.com/startup_jedi
Comments